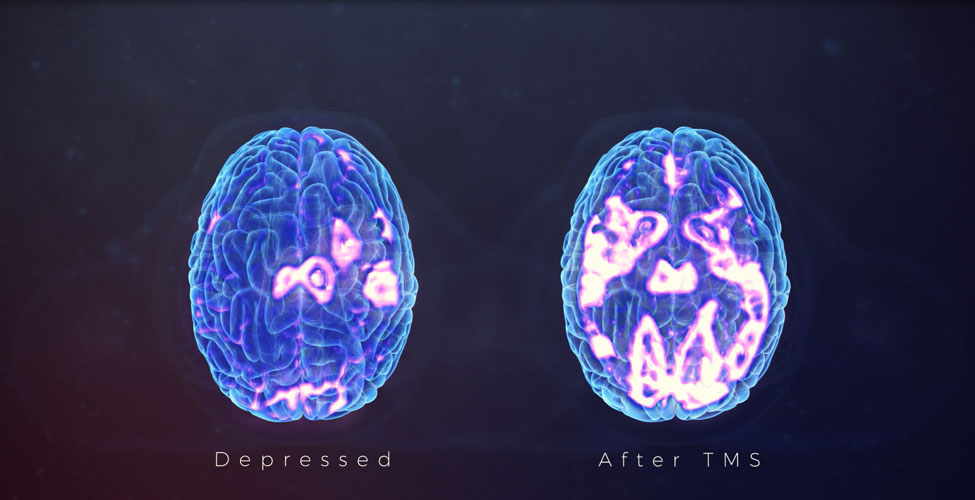
Our team is excited to announce that we are now offering Transcranial Magnetic Stimulation (TMS) therapy as an alternative treatment for patients with treatment resistant depression. TMS therapy is an FDA approved, non-invasive treatment that is effective for reducing or eliminating the symptoms of depression in adults who have not experienced improvement with traditional treatments, including medication or psychotherapy, or for those who cannot tolerate antidepressant medications.Transcranial Magnetic Stimulation (TMS) uses focused magnetic pulses to stimulate nerve cells in the brain to improve symptoms of depression. TMS therapy is thought to activate regions of the brain that have decreased activity in individuals with depression.
The typical course of TMS therapy involves a 30 minute session five days per week for 6 weeks. For this population with a very poor prognosis for further antidepressant therapy, TMS has been effective for 58% of patients treated, with continued improvement for up to a year after completing initial TMS treatment. In most cases patients may continue their medication and continue psychotherapy during and after TMS therapy.
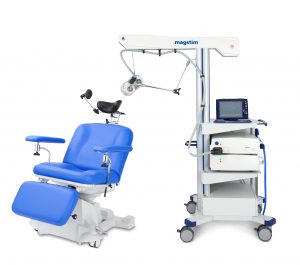

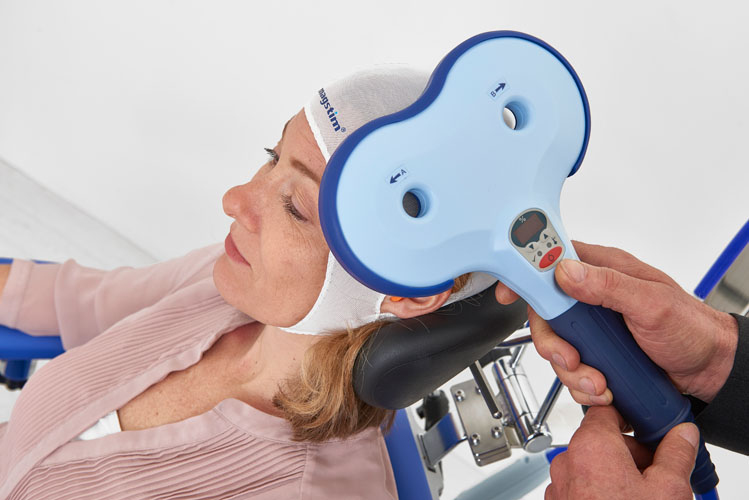

TMS therapy is indicated for adults with major depressive disorder, who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode.
TMS therapy has few known adverse effects, resulting in better patient outcomes.
If you are interested in learning more about this exciting new treatment, call our TMS coordinator at 517-548-1537 for a phone consultation.
Feel free to visit Magstim for Frequently Asked Questions or watch this video: What is TMS?